Directions : In the following question more than one of the answers given may be correct. Select the correct answer and mark it according to the code:
Excess of\[KI\]reacts with\[CuS{{O}_{4}}\]solution and then\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]solution is added to it. The correct statements for this reaction are (1) \[C{{u}_{2}}{{I}_{2}}\]is formed (2)\[Cu{{I}_{2}}\]is formed (3)\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]is oxidised (4) evolved\[{{I}_{2}}\]is oxidised.A) 1, 2 and 3 are correct.
B) 1 and 2 are correct.
C) 2 and 4 are correct.
D) 1 and 3 are correct.
Correct Answer: D
Solution :
\[CuS{{O}_{4}}+KI\xrightarrow[{}]{{}}\underset{unstable}{\mathop{Cu{{I}_{2}}}}\,+{{K}_{2}}S{{O}_{4}}\] \[Cu{{I}_{2}}\xrightarrow{{}}C{{u}_{2}}{{I}_{2}}+{{I}_{2}}\]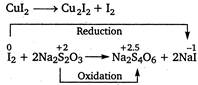 In this reaction\[C{{u}_{2}}{{I}_{2}}\]and\[{{I}_{2}}\]are formed.\[{{I}_{2}}\] reacts with hypo and oxidised it.
In this reaction\[C{{u}_{2}}{{I}_{2}}\]and\[{{I}_{2}}\]are formed.\[{{I}_{2}}\] reacts with hypo and oxidised it.
You need to login to perform this action.
You will be redirected in
3 sec
