Directions : In the following question more than one of the answers given may be correct. Select the correct answer and mark it according to the code:
A gas undergoes the change in its state from position A to position B via three different paths as shown in figure. Select the correct alternative (s).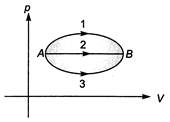 (1) temperature of the gas first increases and then decreases continuously in path 1 (2) heat absorbed/released by the gas is maximum in path 1 (3) change in internal energy in all the three paths is equal (4) in all the three paths heat is absorbed by the gas
(1) temperature of the gas first increases and then decreases continuously in path 1 (2) heat absorbed/released by the gas is maximum in path 1 (3) change in internal energy in all the three paths is equal (4) in all the three paths heat is absorbed by the gas
A) 1, 2 and 3 are correct
B) 1 and 2 are correct
C) 2 and 4 are correct
D) 1 and 3 are correct
Correct Answer: A
Solution :
Internal energy (U) depends only on the initial and final states. Hence,\[\Delta U\]will be same in all the three paths. In all the three paths work done by the gas is positive (volume is increasing) and the product\[pV\]or temperature T is increasing. Therefore, internal energy is also increasing so, from first law of thermodynamics heat will be absorbed by the gas. Further area under p-V graph is maximum in path 1 while\[\Delta U\]is same for all three paths. Therefore, heat absorbed by the gas is maximum in path 1. For temperature of the gas we can see the product \[pV\]which first increases in paht 1 and then decreases us.You need to login to perform this action.
You will be redirected in
3 sec
