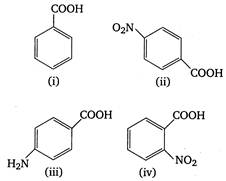
A) (iii) < (i) < (iv) < (ii)
B) (ii) < (iv) < (i) < (iii)
C) (iv) < (ii) < (i) < (iii)
D) (iii) < (i) < (ii) < (iv)
Correct Answer: C
Solution :
\[p{{K}_{a}}\]value is highest for weak acid. Presence of electron withdrawing groups (like \[N{{O}_{2}},Cl\])on benzene nucleus stabilises the benzoate ion by dispersing charge and thus increase acidity. On the other hand, electron releasing groups like\[\text{ }NH{{ & }_{2}},\] destabilises the benzoate ion by intensifying charge and thus decrease acidity or\[{{K}_{a}}\](or increase\[p{{K}_{a}}\]value). Thus, the correct order of \[p{{K}_{a}}\]of given acids is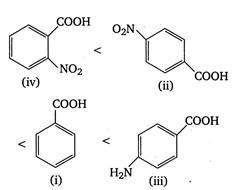
You need to login to perform this action.
You will be redirected in
3 sec
