Directions : In the following question more than one of the answers given may be correct. Select the correct answer and mark it according to the code:
Consider the following compounds.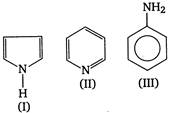 Select the correct statement(s). (1) I is more basic than II (2) II is more basic than I and III (3) III is more basic than II (4) I is weakly acidic
Select the correct statement(s). (1) I is more basic than II (2) II is more basic than I and III (3) III is more basic than II (4) I is weakly acidic
A) 1, 2 and 3 are correct
B) 1 and 2 are correct
C) 2 and 4 are correct
D) 1 and 3 are correct
Correct Answer: C
Solution :
In aniline (III), the lone pairs of nitrogen atom takes part in delocalisation while in pyrrole (I) these are involved in the formation of aromatic sextet. Thus, are not available for donation. Hence, both are less basic. However, pyridine (II) due to the absence of such kind of delocalisation, is more basic than I and III. Further I is so much less basic that with strong bases such as K metal, it behaves as a weak acid.You need to login to perform this action.
You will be redirected in
3 sec
