A) \[C{{H}_{2}}OHC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}COOH\]
B) \[C{{H}_{3}}CH(OH)C{{H}_{2}}COOH\]
C) \[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-COOH\]
D) \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-COOH\]
Correct Answer: A
Solution :
\[\delta \]or\[\gamma -\]hydroxy carboxylic acids readily undergo dehydraction upon heating. Thus,\[C{{H}_{2}}OHC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}COOH\]readily undergoes dehydration.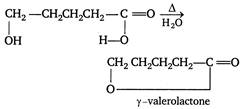
You need to login to perform this action.
You will be redirected in
3 sec
