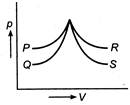
 In which case no exchange of heat occurs with the sample?
In which case no exchange of heat occurs with the sample?
A) P
B) Q
C) R
D) S
Correct Answer: D
Solution :
S represents adiabatic process, while R, an isothermal process. In case of P and Q both pressure and volume increases. This is possible only when the temperature of the system rises and work is done on the system. In other words it gains heat.You need to login to perform this action.
You will be redirected in
3 sec
