Directions : In the following question more than one of the answers given may be correct. Select the correct answer and mark it according to the code:
Which kinds of isomerism is exhibited by octahedral\[Co(N{{H}_{3}})B{{r}_{2}}Cl\]? (1) Optical (2) Geometrical (3) Linkage (4) lonisationA) 1, 2 and 3 are correct
B) 1 and 2 are correct
C) 2 and 4 are correct
D) 1 and 3 are correct
Correct Answer: C
Solution :
Octahedral\[Co{{(N{{H}_{3}})}_{4}}B{{r}_{2}}Cl\]shows ionisation and geometrical isomerism. In ionisation isomerism ligands show different coordination sphere and the anions present outside the coordination sphere. \[[Co{{(N{{H}_{3}})}_{4}}B{{r}_{2}}]Cl{{[Co{{(N{{H}_{3}})}_{4}}B{{r}_{2}}]}^{+}}+C{{l}^{-}}\] \[[Co{{(N{{H}_{3}})}_{4}}BrCl]Br{{[Co{{(N{{H}_{3}})}_{4}}BrCl]}^{+}}+B{{r}^{-}}\] In geometrical isomerism, coordination number of central atom is six and shape is octahedral.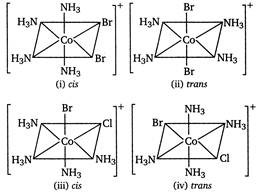
You need to login to perform this action.
You will be redirected in
3 sec
