Directions : In the following question more than one of the answers given may be correct. Select the correct answer and mark it according to the code:
Which species has one lone pair of electrons on the central atom? (1) \[[ClO_{3}^{-}]\] (2) \[Xe{{F}_{4}}\] (3) \[S{{F}_{4}}\] (4) \[[I_{3}^{-}]\]A) 1, 2 and 3 are correct
B) 1 and 2 are correct
C) 2 and 4 are correct
D) 1 and 3 are correct
Correct Answer: D
Solution :
In\[ClO_{3}^{-},Cl\]is central atom. It is sp3 hybrid and on it one lone pair of electrons is present.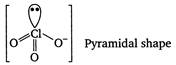 In\[S{{F}_{4}},S\]is central atom. It is sp3d hybrid and on it one lone pair of electrons is present.
In\[S{{F}_{4}},S\]is central atom. It is sp3d hybrid and on it one lone pair of electrons is present. 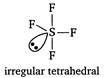
You need to login to perform this action.
You will be redirected in
3 sec
