Directions : In the following question more than one of the answers given may be correct. Select the correct answer and mark it according to the code:
Which of the following are oxidised by\[{{O}_{3}}\]? (1)\[KI\] (2) \[FeS{{O}_{4}}\] (3) \[{{K}_{2}}Mn{{O}_{4}}\] (4) \[KMn{{O}_{4}}\]A) 1, 2 and 3 are correct
B) 1 and 2 are correct
C) 2 and 4 are correct
D) 1 and 3 are correct
Correct Answer: A
Solution :
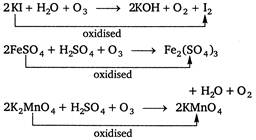 \[KMn{{O}_{4}}+{{O}_{3}}\xrightarrow{{}}No\text{ }reaction\] because in KMn04 oxidation state of\[Mn\] is + 7. Hence, it is the highest oxidation state of Mn. So,\[KMn{{O}_{4}}\]is not oxidised by ozones.
\[KMn{{O}_{4}}+{{O}_{3}}\xrightarrow{{}}No\text{ }reaction\] because in KMn04 oxidation state of\[Mn\] is + 7. Hence, it is the highest oxidation state of Mn. So,\[KMn{{O}_{4}}\]is not oxidised by ozones.
You need to login to perform this action.
You will be redirected in
3 sec
