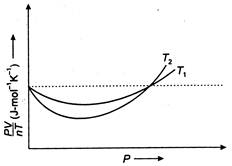
 Read the following statements concerning the above curves: (i) The dotted line corresponds to the 'ideal' gas behaviour. (ii)\[{{T}_{1}}>{{T}_{2}}\] (iii) The value of\[\frac{pV}{nT}\]at the point where the curves meet on the y-axis is the same for all gases. Which of the above statement is true?
Read the following statements concerning the above curves: (i) The dotted line corresponds to the 'ideal' gas behaviour. (ii)\[{{T}_{1}}>{{T}_{2}}\] (iii) The value of\[\frac{pV}{nT}\]at the point where the curves meet on the y-axis is the same for all gases. Which of the above statement is true?
A) (i) only
B) (i) and (ii) only
C) All of these
D) None of these
Correct Answer: C
Solution :
(i) The dotted line in the diagram shows that there is no deviation in the value of\[\frac{PV}{nT}\]for different temperature\[{{T}_{1}}\]and\[{{T}_{2}}\]for increasing pressure, so this gas behaves ideally. Hence, dotted line corresponds to 'idea? Gas. behaviour. (ii) We know at high temperature, the deviation of the gas is less and at low temperature the deviation of gas is more. In the graph, deviation for\[{{T}_{2}}\]is greater than for \[{{T}_{1}}\].Thus, \[{{T}_{1}}>{{T}_{2}}\] (iii) Since, the two curves intersect at dotted line, so the value of\[\frac{PV}{nT}\]at that point on the y-axis is same for all gases. Thus, option is true.You need to login to perform this action.
You will be redirected in
3 sec
