A) A and R both are correct and R is the correct explanation of A.
B) A and R both are correct but R is not the correct explanation of A.
C) A is incorrect while R is correct.
D) A and R both are incorrect.
Correct Answer: A
Solution :
In complex\[{{[Co{{F}_{6}}]}^{3-}}\]oxidation number of\[Co=+3\] Electronic configuration of \[C{{o}^{3+}}=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}},3{{p}^{6}},3{{d}^{6}}\] In \[C{{o}^{3+}}\]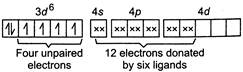 So,\[{{[Co{{F}_{6}}]}^{3-}}\]is paramagnetic. \[C{{o}^{3+}}\]has\[3{{d}^{6}}\]outer electronic configuration, but four unpaired electrons don't pair up because of weak field provided by\[{{F}^{-}}\]. Hence, assertion is correct and reason is the correct explanation of assertion
So,\[{{[Co{{F}_{6}}]}^{3-}}\]is paramagnetic. \[C{{o}^{3+}}\]has\[3{{d}^{6}}\]outer electronic configuration, but four unpaired electrons don't pair up because of weak field provided by\[{{F}^{-}}\]. Hence, assertion is correct and reason is the correct explanation of assertion
You need to login to perform this action.
You will be redirected in
3 sec
