A) Primary alcohols are easily oxidised to aldehydes, which are oxidised to acids with the same number of C-atoms.
B) Secondary alcohols are easily oxidised to ketones, which are oxidised to acids with the same number of C-atoms.
C) Secondary alcohols are easily oxidised to ketones, which are oxidised to acids with lesser number of C-atoms.
D) Secondary and tertiary alcohols on oxidation form acids with lesser number of C-atoms.
Correct Answer: B
Solution :
Primary alcohols are easily oxidised to aldehyde and then to acid, both containing the same number of carbon atom, while secondary alcohol are easily oxidised to ketone with same number of carbon atoms, but ketone oxidized to carboxylic acid containing lesser number of carbon atoms than original alcohol. \[\underset{\begin{smallmatrix} \,\,\,\,ethyl \\ alcohol \end{smallmatrix}}{\mathop{C{{H}_{3}}C{{H}_{2}}}}\,-OH+[O]\xrightarrow[-{{H}_{2}}O]{{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}_{2}}S{{O}_{4}}}\] \[\underset{acetaldehyde}{\mathop{C{{H}_{3}}CHO}}\,\xrightarrow{[O]}\underset{acetic\,acid}{\mathop{C{{H}_{3}}COOH}}\,\]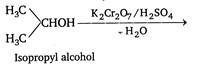
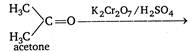 \[\underset{acetic\,acid}{\mathop{C{{H}_{3}}COOH}}\,+C{{O}_{2}}+{{H}_{2}}O\] Thus, all the alcohols on oxidation finally give acids but acids obtained from secondary and tertiary alcohols contain lesser number of carbon atom.
\[\underset{acetic\,acid}{\mathop{C{{H}_{3}}COOH}}\,+C{{O}_{2}}+{{H}_{2}}O\] Thus, all the alcohols on oxidation finally give acids but acids obtained from secondary and tertiary alcohols contain lesser number of carbon atom.
You need to login to perform this action.
You will be redirected in
3 sec
