A) 120 cc
B) 22.4 cc
C) 11.2cc
D) 44.8 cc
Correct Answer: B
Solution :
\[2{{H}_{2}}O4{{H}^{+}}+2{{O}^{2-}}\]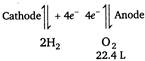 \[\because \]\[4\times 96500\text{ }C\]electricity liberate\[{{O}_{2}}\] \[=22400\text{ }cc\] \[\therefore \]\[(2\times 193)C\]electricity will liberate\[{{O}_{2}}\] \[=\frac{22400\times 386}{4\times 96500}\] \[=22.4cc\]
\[\because \]\[4\times 96500\text{ }C\]electricity liberate\[{{O}_{2}}\] \[=22400\text{ }cc\] \[\therefore \]\[(2\times 193)C\]electricity will liberate\[{{O}_{2}}\] \[=\frac{22400\times 386}{4\times 96500}\] \[=22.4cc\]
You need to login to perform this action.
You will be redirected in
3 sec
