A) 31.6
B) 52.7
C) 7.0
D) 158.0
Correct Answer: D
Solution :
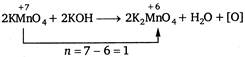 Equivalent wt. of\[KMn{{O}_{4}}=\frac{molecular\text{ }weight}{n}\] \[=\frac{158}{1}=158.0\]
Equivalent wt. of\[KMn{{O}_{4}}=\frac{molecular\text{ }weight}{n}\] \[=\frac{158}{1}=158.0\]
You need to login to perform this action.
You will be redirected in
3 sec
