A) square planar
B) trigonal
C) trigonal planar
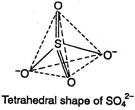
D) tetrahedral
Correct Answer: D
Solution :
Sulphate ion \[(SO_{4}^{2-})\] has tetrahedral geometry, as in it, S-atom undergoes \[s{{p}^{3}}-\]hybridisation. \[S=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{4}}\] S in II excited state =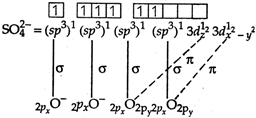
You need to login to perform this action.
You will be redirected in
3 sec
