A) 18
B) 68
C) 34
D) (d 17
Correct Answer: D
Solution :
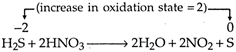 Hence, the equivalent weight of \[{{H}_{2}}S=\frac{Molecular\,weight}{change\,in\,oxidation\,number}\] \[=\frac{34}{2}\] \[=17\]
Hence, the equivalent weight of \[{{H}_{2}}S=\frac{Molecular\,weight}{change\,in\,oxidation\,number}\] \[=\frac{34}{2}\] \[=17\]
You need to login to perform this action.
You will be redirected in
3 sec
