A) 4
B) 5
C) 6
D) 7
Correct Answer: D
Solution :
The isomers of the compound having molecular formula \[{{C}_{4}}{{H}_{10}}O\] are as: (i)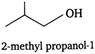 (iii)
(iii) 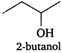 (iv)
(iv) 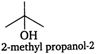 (v)
(v) 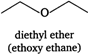 (vi)
(vi) 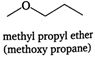 (vii)
(vii) You need to login to perform this action.
You will be redirected in
3 sec
