A) \[s{{p}^{3}}\]
B) \[s{{p}^{2}}\]
C) \[s{{p}^{3}}d\]
D) \[s{{p}^{2}}d\]
E) \[s{{p}^{3}}{{d}^{2}}\]
Correct Answer: C
Solution :
The hybridization of xenon in\[Xe{{F}_{2}}\]is\[s{{p}^{3}}d\]. The presence of three lone pair gives linear shape to the molecule and bond angle is\[180{}^\circ \].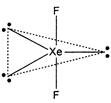
You need to login to perform this action.
You will be redirected in
3 sec
