A) linear with +ve slope and zero intercept
B) linear with -ve slope and zero intercept
C) linear with -ve slope and non-zero intercept
D) linear with +ve slope and non-zero intercept
E) a curve asymptotic to concentration axis
Correct Answer: C
Solution :
For a zero order reaction, the plot of concentration of reactant\[vs\]time is a straight line (linear) with a negative slope and non-zero intercept.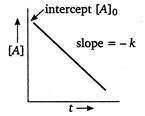
You need to login to perform this action.
You will be redirected in
3 sec
