1. 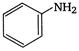 |
2. 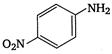 |
3. 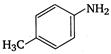 |
4.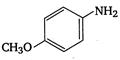 |
5. 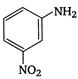 |
A) \[2<5<1<3<4\]
B) \[5<2<1<3<4\]
C) \[2<5<1<4<3\]
D) \[5<2<1<4<3\]
E) \[2<5<4<3<1\]
Correct Answer: A
Solution :
\[OC{{H}_{3}}\]is strongest electron releasing group (+M effect) which opposes most the dispersion of lone pair of electron of nitrogen into the ring. Thus\[OC{{H}_{3}}\]being at para- position imparts highest basicity.\[N{{O}_{2}}\]being at meta- position stabilizes the electron pair of nitrogen only by\[-I\]effect. While N03 being present at para- position due to -M effect and \[-I\]effect stabilizes the lone pair of electron of nitrogen, most and impart least basicity.
You need to login to perform this action.
You will be redirected in
3 sec
