| I.\[NC{{l}_{5}}\]does not exist while\[PC{{l}_{5}}\]does |
| II. Lead prefers to form tetravalent compounds |
| III. The three\[CO\]bonds are not equal in the carbonate ion |
| IV. Both\[O_{2}^{+}\]and\[NO\]are paramagnetic |
A) I, III and IV
B) I and IV
C) II and III
D) I and III
E) IV only
Correct Answer: C
Solution :
(I) In nitrogen d-orbitals are absent, so it does not form\[NC{{l}_{5}}\]. Thus,\[NC{{l}_{5}}\]does not exist but \[PC{{l}_{5}}\]does. (II)\[P{{b}^{2+}}\]is more stable than\[P{{b}^{4+}},\]due to inert pair effect. (Ill) In carbonate ion\[(CO_{3}^{2-})\]all the three\[C-O\]bonds are identical due to resonance.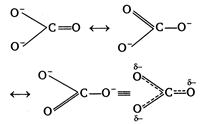 (IV) \[O_{2}^{+}(8+8-1=15)=\sigma 1{{s}^{2}},{{\sigma }^{*}}1{{s}^{2}},\sigma 2{{s}^{2}},\] \[{{\sigma }^{*}}2{{s}^{2}},\] \[\sigma 2p_{x}^{2},\pi 2p_{y}^{2}\approx 2p_{z}^{2},\overset{*}{\mathop{\pi }}\,2p_{y}^{1}\] \[NO(7+8=15)\] Hence, both\[O_{2}^{+}\]and\[NO\]contains one unpaired electron, so paramagnetic.
(IV) \[O_{2}^{+}(8+8-1=15)=\sigma 1{{s}^{2}},{{\sigma }^{*}}1{{s}^{2}},\sigma 2{{s}^{2}},\] \[{{\sigma }^{*}}2{{s}^{2}},\] \[\sigma 2p_{x}^{2},\pi 2p_{y}^{2}\approx 2p_{z}^{2},\overset{*}{\mathop{\pi }}\,2p_{y}^{1}\] \[NO(7+8=15)\] Hence, both\[O_{2}^{+}\]and\[NO\]contains one unpaired electron, so paramagnetic.
You need to login to perform this action.
You will be redirected in
3 sec
