| List - I | List - II |
| (A) \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] | 1. \[T{{i}^{4+}}\] |
| (B) Chlorophyll | 2.\[s{{p}^{3}};\]paramagnetic |
| (C) Ziegler - Natta catalyst | 3. non-planar |
| (D) \[{{[NiC{{l}_{4}}]}^{2-}}\] | 4. \[M{{g}^{2+}}\] |
| (E) Deoxyhaemoriobin | 5. Planar |
| 6.\[ds{{p}^{2}};\] diamagnetic |
A) A-6 B-4 C-1 D-2 E-3
B) A-2 B-4 C-1 D-6 E-3
C) A-2 B-4 C-1 D-6 E-5
D) A-6 B-4 C-1 D-2 E-5
E) A-2 B-4 C-3 D-6 E-5
Correct Answer: A
Solution :
(a) \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] \[Ni(28)=[Ar]\,3{{d}^{8}}4{{s}^{2}}\] \[N{{i}^{2+}}=[Ar]\,3{{d}^{8}},4s\]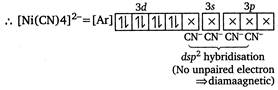 (b) Chlorophyll contains\[M{{g}^{2+}}\]ion. (c) Ziegler-Natta catalyst is\[T{{i}^{4+}}+{{({{C}_{2}}{{H}_{5}})}_{3}}Al\]. (d) \[{{[NiC{{l}_{4}}]}^{2-}}\] In this case,\[C{{l}^{-}}\]is a weak field ligand, so does not couse pairing. Hence,
(b) Chlorophyll contains\[M{{g}^{2+}}\]ion. (c) Ziegler-Natta catalyst is\[T{{i}^{4+}}+{{({{C}_{2}}{{H}_{5}})}_{3}}Al\]. (d) \[{{[NiC{{l}_{4}}]}^{2-}}\] In this case,\[C{{l}^{-}}\]is a weak field ligand, so does not couse pairing. Hence, 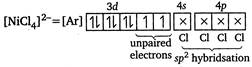 Due to the presence of unpaired electrons, it is paramagnetic. (e) Deoxyhaemoglobin is non-planar while oxyhaemoglobin is planar. Hence, the correct matching is as \[A-6;B-4;C-1;D-2;E-3\]
Due to the presence of unpaired electrons, it is paramagnetic. (e) Deoxyhaemoglobin is non-planar while oxyhaemoglobin is planar. Hence, the correct matching is as \[A-6;B-4;C-1;D-2;E-3\]
You need to login to perform this action.
You will be redirected in
3 sec
