A) \[{{[Co{{F}_{6}}]}^{3-}}\]
B) \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
C) \[{{[Ni{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
D) \[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
E) \[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
Correct Answer: A
Solution :
(i) In\[{{[Co{{F}_{6}}]}^{3-}}\]ion, the oxidation state of cobalt is +3. \[C{{o}^{3+}}\]ion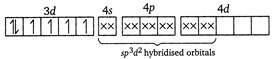 Here,\[{{F}^{-}}\]ion provides a weak ligand field and is unable to pair up the unpaired electrons of the 3d-orbitals. Thus, it is highly paramagnetic. (ii) In\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]ion, \[N{{H}_{3}}\] provides a strong field ligand and the electrons of metal are made to pair up, so the complex will be diamagnetic. Formation of\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
Here,\[{{F}^{-}}\]ion provides a weak ligand field and is unable to pair up the unpaired electrons of the 3d-orbitals. Thus, it is highly paramagnetic. (ii) In\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]ion, \[N{{H}_{3}}\] provides a strong field ligand and the electrons of metal are made to pair up, so the complex will be diamagnetic. Formation of\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] 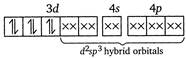 (iii) Similarly in\[{{[Ni{{(N{{H}_{3}})}_{4}}]}^{2+}},N{{H}_{3}}\]is a strong field ligand and the complex is diamagnetic. \[N{{i}^{2+}}\]ion:
(iii) Similarly in\[{{[Ni{{(N{{H}_{3}})}_{4}}]}^{2+}},N{{H}_{3}}\]is a strong field ligand and the complex is diamagnetic. \[N{{i}^{2+}}\]ion: 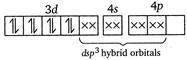 (iv)\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]has Ni in the + 2 oxidation state and\[ds{{p}^{2}}\]hybridisation. Thus it is also diamagnetic. (v) In\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]ions, oxidation state of\[Fe\]is +2 and\[C{{N}^{-}}\]ligand is a strong field ligand. Thus the resulting complex ion involves\[{{d}^{2}}s{{p}^{3}}\]hybridisation and diamagnetic as it does not contain any unpaired electrons. \[F{{e}^{2+}}\]ion:
(iv)\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]has Ni in the + 2 oxidation state and\[ds{{p}^{2}}\]hybridisation. Thus it is also diamagnetic. (v) In\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]ions, oxidation state of\[Fe\]is +2 and\[C{{N}^{-}}\]ligand is a strong field ligand. Thus the resulting complex ion involves\[{{d}^{2}}s{{p}^{3}}\]hybridisation and diamagnetic as it does not contain any unpaired electrons. \[F{{e}^{2+}}\]ion: 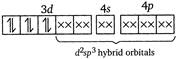 \[\because \]Magnetic moment \[=\sqrt{n(n+2)}\] where, \[n=\]no. of unpaired electrons \[\therefore \]\[{{[Co{{F}_{6}}]}^{3-}}\]has maximum magnetic moment.
\[\because \]Magnetic moment \[=\sqrt{n(n+2)}\] where, \[n=\]no. of unpaired electrons \[\therefore \]\[{{[Co{{F}_{6}}]}^{3-}}\]has maximum magnetic moment.
You need to login to perform this action.
You will be redirected in
3 sec
