A) acetylene and ethylene
B) acetylene and benzene
C) ethylene and benzene
D) toluene and benzene
E) benzene and styrene
Correct Answer: B
Solution :
The percentage composition of compounds X and Y \[\begin{align} & \,\,\,\,\,C~~~~~~~~~~~\,\,\,\,\,:~~~~~\,\,\,\,\,\,\,H \\ & \frac{12}{13}\times 100%\,\,\,\,\,\,:\,\,\,\,\,\,\,\,\,\,\,\frac{1}{13}\times 100% \\ \end{align}\] i.e., the ratio of masses of C and H in the organic compounds \[\begin{align} & C~~~~~:~~~~~H \\ & \frac{12}{13}\,\,\,\,\,:\,\,\,\,\,\,\,\frac{1}{13} \\ \end{align}\] or \[12:1\] Thus, the empirical formula of the compounds X and V is CH and X decolorize bromine water while Y does not. Thus, X and Y must be acetylene\[(CH\equiv CH)\]and benzene\[({{C}_{6}}{{H}_{6}})\] respectively.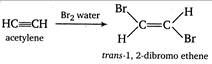 \[\xrightarrow[{}]{B{{r}_{2}}\,water}\underset{1,\text{ }1,\text{ }2\text{ },2-tetrabromoethane}{\mathop{H-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}}\,-H}}\,\]
\[\xrightarrow[{}]{B{{r}_{2}}\,water}\underset{1,\text{ }1,\text{ }2\text{ },2-tetrabromoethane}{\mathop{H-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}}\,-H}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
