A) four equivalent\[Cr-O\]bonds only
B) si equivalent\[Cr-O\]bonds and one \[O-O\]bond
C) six equivalent\[Cr-O\]bonds and one \[Cr-Cr\]bond
D) eight equivalent\[Cr-O\]bonds
E) six equivalent\[Cr-O\]bonds and one \[Cr-O-Cr\]bond
Correct Answer: E
Solution :
In dichromate ion\[(C{{r}_{2}}O_{7}^{2-})\]two tetrahedral units are shared through an oxygen atom. The two\[CrO\]bonds which share an oxygen atom are longer than other six equivalent \[CrO\]bonds.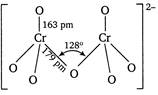
You need to login to perform this action.
You will be redirected in
3 sec
