A) trichloro ethane
B) dichloro acetone
C) dichloro acetaldehyde
D) trichloro acetaldehyde
Correct Answer: D
Solution :
D.D.T. is dichloro-diphenyl trichloro- ethane. It can be prepared by treating chlorobenzene with chloral \[\underset{\text{Chlorobenzene}}{\mathop{2{{C}_{6}}{{H}_{5}}Cl}}\,+\underset{\begin{smallmatrix} \text{Trichloro} \\ \text{acetaldehyde} \end{smallmatrix}}{\mathop{CC{{l}_{3}}CHO}}\,\xrightarrow{con.{{H}_{2}}S{{O}_{4}}}CC{{l}_{3}}CH\]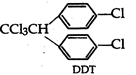
You need to login to perform this action.
You will be redirected in
3 sec
