A) \[+R-effect\]of \[-N{{H}_{2}}\] group
B) \[-I\,effect\] of \[-N{{H}_{2}}\] group
C) \[-\operatorname{Re}\,effect\]of \[-N{{H}_{2}}\] group
D) hyper conjugation effect
Correct Answer: A
Solution :
\[-N{{H}_{2}}\] has \[+R\] effect, it donates electrons to the benzene ring. As a result, the lone pair of electron on the N-atom gets delocalized over the benzene ring and thus it is less readily available for protonation. Hence, aniline is a weaker base than cyclohexylamine.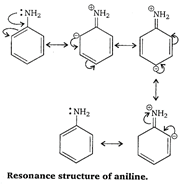
You need to login to perform this action.
You will be redirected in
3 sec
