A) \[1\]
B) \[2\]
C) \[3\]
D) \[4\]
Correct Answer: D
Solution :
One water molecule is joined to four water molecules - two with H-atoms and other two with O-atoms. Thus, the maximum number of hydrogen bonds that a molecule of water can have is four as shown below: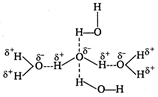
You need to login to perform this action.
You will be redirected in
3 sec
