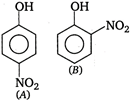
A) higher than that of [A]
B) lower than that of [A]
C) higher or lower than [A] , depending on the size of the vessel
D) same as that of [A]
Correct Answer: A
Solution :
In [A] para-nitro phenol intermolecular (between two molecules) H-bonding exists while in [B] ortho- nitro phenol, intramolecular H-bonding exists. Because of the presence of intramolecular H-bonding, the boiling point of [B] is lower as compared to [A] and thus, [B] is more volatile, ie, has higher vapour pressure as compared to [A].You need to login to perform this action.
You will be redirected in
3 sec
