A) It forms only one type of monosubstituted product
B) There are three carbon-carbon single bonds and three carbon-carbon double bonds
C) The heat of hydrogenation of benzene is less than the theoretical value
D) The bond angle between the carbon-carbon bonds is \[{{120}^{o}}\]
Correct Answer: B
Solution :
The structure of benzene is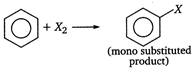
You need to login to perform this action.
You will be redirected in
3 sec
