A) \[CO,C{{O}_{2}},HCO_{2}^{-},CO_{3}^{2-}\]
B) \[C{{O}_{2}},HCO_{2}^{-},CO,CO_{3}^{2-}\]
C) \[CO_{3}^{2-},HCO_{2}^{-},C{{O}_{2}},CO\]
D) \[CO,CO_{3}^{2-},C{{O}_{2}},HCO_{2}^{-}\]
Correct Answer: C
Solution :
: Bond order \[\propto \frac{1}{Bond\,length}\] Bond length \[=\frac{Bond\,order\,of\,each\,C-O\,bond}{Total\,no.\,of\,resontating\,structures}\] [a] \[CO\to C\overset{\leftarrow }{\mathop{=}}\,O\] \[\Rightarrow \] \[\frac{2+1}{1}=3.0\] [b] \[C{{O}_{2}}\to O=C=O\] \[\Rightarrow \] \[\frac{2+2}{2}=2.0\] [c]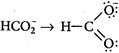 \[\Rightarrow \] \[\frac{2+1}{2}=1.5\] [d]
\[\Rightarrow \] \[\frac{2+1}{2}=1.5\] [d] You need to login to perform this action.
You will be redirected in
3 sec
