A) \[ii<iv<iii<i\]
B) \[ii<iii<i<iv\]
C) \[iii<ii<i<iv\]
D) \[ii<iii<iv<i\]
Correct Answer: D
Solution :
: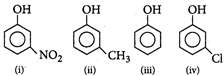 Nitro group has both -R effect and -J effect, but -R effect predominates. Due to stronger electron withdrawing nature of -N02 group, phenoxide ion is stabilized more. Hence nitrophenol is more acidic than phenol. Methyl group destabilizes the phenoxide ion by +I effect and hyper conjugation. Hence w-cresol is weaker acid than phenol. Chlorine have both +R and -I effect, but -I effect predominates. Hence w-chlorophenol is more acidic than phenol. -R effect of nitro group is stronger than -I effect of chlorine, hence m-nitrophenol is more acidic than m-chlorophenol. Therefore the correct order of acidic strength is m-nitrophenol > m-chlorophenol > phenol > m-cresol
Nitro group has both -R effect and -J effect, but -R effect predominates. Due to stronger electron withdrawing nature of -N02 group, phenoxide ion is stabilized more. Hence nitrophenol is more acidic than phenol. Methyl group destabilizes the phenoxide ion by +I effect and hyper conjugation. Hence w-cresol is weaker acid than phenol. Chlorine have both +R and -I effect, but -I effect predominates. Hence w-chlorophenol is more acidic than phenol. -R effect of nitro group is stronger than -I effect of chlorine, hence m-nitrophenol is more acidic than m-chlorophenol. Therefore the correct order of acidic strength is m-nitrophenol > m-chlorophenol > phenol > m-cresol
You need to login to perform this action.
You will be redirected in
3 sec
