A) \[2\]
B) \[6\]
C) \[12\]
D) \[10\]
Correct Answer: D
Solution :
For a given value of n, possible values of \[l=0\] to \[(n-1)\]. Each value of \[l\] corresponds to a sub-energy level. The value of \[l=0,1,2,3\]are also represented by the letters s, p, d, f respectively. \[l=0\]or s-subshell \[l=1\] or p-subshell \[l=2\] or d-subshell \[l=3\] or/-subshell Thus, for M shell \[n=3\] and \[l=0,1,2\] This shows that third orbital (M-shell) contains three subshells \[(3s,3p,3d)\]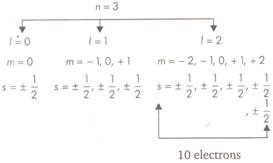 So, in the given question, the maximum number of electrons in an atom with \[l=2\]and \[n=3\]is 10.
So, in the given question, the maximum number of electrons in an atom with \[l=2\]and \[n=3\]is 10.
You need to login to perform this action.
You will be redirected in
3 sec
