A) \[PC{{l}_{3}}\]
B) \[S{{O}_{3}}\]
C) \[CO_{3}^{2-}\]
D) \[NO_{3}^{-}\]
Correct Answer: A
Solution :
Key Idea: (i) Find the hybridisation of the molecule by using following formula: \[H=\frac{1}{2}(V+X-C+A)\] where, H = hybridisation V = number of electrons in valence shell of central atom X = number of monovalent atoms C = charge on cation A = charge on onion (ii) Shape is determined by knowing hybridisation and lone pair of electrons in valence shell of central atom.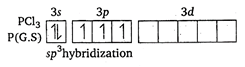 The shape is pyramidal due to presence of one lone pair of electron. \[S{{O}_{3}}\] \[H=\frac{1}{2}(6+0-0+0)\] = 3 \[\therefore \] \[s{{p}^{2}}\] hybridisation and triangular planar shape. \[C{{O}_{3}}^{2-}\] \[H=\frac{1}{2}(4+0+2-0)\] or \[H=3\] \[\therefore \] \[s{{p}^{2}}\] hybridisation and triangular planar shape. \[N{{O}_{3}}^{-}\] \[H=\frac{1}{2}=(5+0-0+1)\] = 3 \[\therefore \] \[s{{p}^{2}}\] hybridisation and triangular planar shape.
The shape is pyramidal due to presence of one lone pair of electron. \[S{{O}_{3}}\] \[H=\frac{1}{2}(6+0-0+0)\] = 3 \[\therefore \] \[s{{p}^{2}}\] hybridisation and triangular planar shape. \[C{{O}_{3}}^{2-}\] \[H=\frac{1}{2}(4+0+2-0)\] or \[H=3\] \[\therefore \] \[s{{p}^{2}}\] hybridisation and triangular planar shape. \[N{{O}_{3}}^{-}\] \[H=\frac{1}{2}=(5+0-0+1)\] = 3 \[\therefore \] \[s{{p}^{2}}\] hybridisation and triangular planar shape.
You need to login to perform this action.
You will be redirected in
3 sec
