A) Tartaric acid
B) Glycerol
C) Ethylene glycol
D) Oxalic acid
Correct Answer: A
Solution :
Key Idea: For a compound to be optically active. (i) It must have at least one asymmetric or chiral carbon atom. (ii) The compound must not have plane of symmetry. Draw the structure of compounds, given in choices to find the correct answer. [a]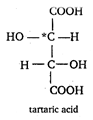 It has two asymmetric carbon atoms and no plane of symmetry. \[\therefore \] It is optically active. [b] \[\begin{align} & C{{H}_{2}}OH \\ & | \\ & CHOH \\ & | \\ & C{{H}_{2}}OH \\ \end{align}\] None of the C atom is asymmetric. \[\therefore \] Optically inactive. [c] \[\underset{\begin{smallmatrix} | \\ C{{H}_{2}}OH \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}OH\] \[\because \] It has no asymmetric carbon atom. \[\therefore \] Optically inactive. [d] \[\underset{\begin{smallmatrix} | \\ COOH \end{smallmatrix}}{\mathop{C}}\,OOH\] \[\because \] It has no asymmetric carbonlatom. \[\therefore \] optically inactive.
It has two asymmetric carbon atoms and no plane of symmetry. \[\therefore \] It is optically active. [b] \[\begin{align} & C{{H}_{2}}OH \\ & | \\ & CHOH \\ & | \\ & C{{H}_{2}}OH \\ \end{align}\] None of the C atom is asymmetric. \[\therefore \] Optically inactive. [c] \[\underset{\begin{smallmatrix} | \\ C{{H}_{2}}OH \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}OH\] \[\because \] It has no asymmetric carbon atom. \[\therefore \] Optically inactive. [d] \[\underset{\begin{smallmatrix} | \\ COOH \end{smallmatrix}}{\mathop{C}}\,OOH\] \[\because \] It has no asymmetric carbonlatom. \[\therefore \] optically inactive.
You need to login to perform this action.
You will be redirected in
3 sec
