A) \[S{{n}^{2+}}\]
B) \[S{{n}^{4+}}\]
C) As
D) \[SAs{{O}_{2}}\]
Correct Answer: D
Solution :
Key Idea: Oxidising agent itself undergoes reduction during a redox reaction.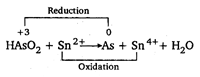 Oxidation state of As in \[HAs{{O}_{2}}\] is \[=x\] \[\therefore \] \[1+x+(2\times -2)=0\] or \[x=3\] Oxidation state of \[As=0\] Oxidation state of Sn in \[S{{n}^{2+}}=+2\] Oxidation state of Sn in \[S{{n}^{4+}}=+4\] \[\therefore \] Increase in oxidation number is oxidation. \[\therefore \] \[S{{n}^{2+}}\] is oxidised to \[S{{n}^{4+}}\] during reaction and it is reducing agent. \[\because \] Decrease in oxidation number is reduction. \[\therefore \] \[HAs{{O}_{2}}\] is reduced to As and it is oxidising agent.
Oxidation state of As in \[HAs{{O}_{2}}\] is \[=x\] \[\therefore \] \[1+x+(2\times -2)=0\] or \[x=3\] Oxidation state of \[As=0\] Oxidation state of Sn in \[S{{n}^{2+}}=+2\] Oxidation state of Sn in \[S{{n}^{4+}}=+4\] \[\therefore \] Increase in oxidation number is oxidation. \[\therefore \] \[S{{n}^{2+}}\] is oxidised to \[S{{n}^{4+}}\] during reaction and it is reducing agent. \[\because \] Decrease in oxidation number is reduction. \[\therefore \] \[HAs{{O}_{2}}\] is reduced to As and it is oxidising agent.
You need to login to perform this action.
You will be redirected in
3 sec
