A) A = acetaldehyde B = 1-pentanal
B) \[A={{C}_{6}}{{H}_{5}}C{{H}_{2}}CHO\] B = 3-pentanone
C) A = formaldehyde B = 2-pentanone
D) A = propionaldehyde B = 1-pentanol
Correct Answer: C
Solution :
According to Cannizaro, aldehyde or ketones having no any a-hydrogen atoms undergo intermolecular oxidation-reduction to form an alcohol and acid. Again, in iodoform test, any alcohol, aldehyde or ketone, when treated with iodine \[({{I}_{2}})\] and sodium hydroxide (NaOH) give iodoform. Therefore, the correct combination of A and B must be formaldehyde and 2-pentanone. Their reactions are as follows : (i) \[\underset{\begin{smallmatrix} formaldehyde \\ (B) \end{smallmatrix}}{\mathop{2HCHO}}\,+NaOH\xrightarrow{{}}C{{H}_{3}}OH\] (ii) \[NaOH+{{I}_{2}}\xrightarrow{{}}NaOI+NaI+{{H}_{2}}O\]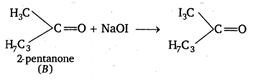 \[\xrightarrow{NaOH}{{C}_{3}}{{H}_{7}}COONa+\underset{iodoform}{\mathop{CH{{I}_{3}}}}\,\]
\[\xrightarrow{NaOH}{{C}_{3}}{{H}_{7}}COONa+\underset{iodoform}{\mathop{CH{{I}_{3}}}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
