A) \[C{{l}_{2}}\]
B) \[{{F}_{2}}\]
C) K
D) Cs
Correct Answer: A
Solution :
The oxidation number of alkali metals (K and Rb) is always +1. Thus, these can not undergo disproportionation reaction. Among halogens \[C{{l}_{2}},\,B{{r}_{2}}\] and \[{{I}_{2}}\] can undergo disproportionation reaction but \[{{F}_{2}}\] does not. The reason for this anomalous behaviour is that Pa being the strongest oxidising agent does not show positive oxidation state.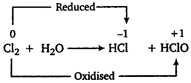
You need to login to perform this action.
You will be redirected in
3 sec
