A)
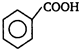
B)
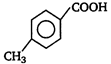
C)
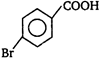
D)
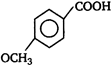
Correct Answer: D
Solution :
Equilibrium constant, \[{{K}_{c}}\] is a function of temperature only. It is independent of concentration of reactants and addition of catalyst or inert gas.You need to login to perform this action.
You will be redirected in
3 sec
