A) XeFO
B) \[Xe{{F}_{2}}{{O}_{2}}\]
C) \[Xe{{F}_{3}}{{O}_{2}}\]
D) \[Xe{{F}_{2}}{{O}_{3}}\]
Correct Answer: D
Solution :
Hybridisation of xenon atom in XeF203.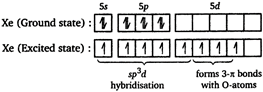 \[\therefore \] The shape of \[Xe{{F}_{2}}{{O}_{3}}\]is trigonal bipyramidal with five \[\sigma \] bonds and three \[\pi \]bonds.
\[\therefore \] The shape of \[Xe{{F}_{2}}{{O}_{3}}\]is trigonal bipyramidal with five \[\sigma \] bonds and three \[\pi \]bonds. 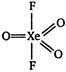
You need to login to perform this action.
You will be redirected in
3 sec
