A) linear
B) planar triangle
C) F-shaped
D) octahedral
Correct Answer: C
Solution :
The central atom in water, oxygen is in a state of \[s{{p}^{2}}-\]hybridization. The four \[s{{p}^{3}}\]orbitals should be tetrahedral mutually inclined at an angle of \[{{109}^{o}}\text{ }28,\] out of the four hybrid orbitals, two singly filled orbitals overlap with s orbitals of hydrogen forming two 0?H bonds. Remaining two non-bonding orbitals each containing a lone pair, occupy more space and compress the two bond pairs. The bond angle decreases from \[{{109}^{o}}\text{ }28\]to \[{{104.5}^{o}}.\] Therefore, water molecule is V shaped with bond angle \[\text{104}\text{.}{{\text{5}}^{\text{o}}}\text{.}\]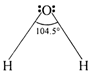
You need to login to perform this action.
You will be redirected in
3 sec
