A) \[{{C}_{2}}{{H}_{6}}\]and \[C{{O}_{2}}\]
B) \[{{C}_{2}}{{H}_{4}}\]and \[C{{O}_{2}}\]
C) \[C{{H}_{4}}\]and \[{{H}_{2}}\]
D) \[C{{H}_{4}}\]and \[C{{O}_{2}}\]
Correct Answer: A
Solution :
This is an example ofKolbes electrolysis. electrolysis , \[2C{{H}_{3}}COOK\xrightarrow{electrolysis}2C{{H}_{3}}CO{{O}^{-}}+2{{K}^{+}}\] At cathode: \[2{{K}^{+}}+2{{e}^{-}}\xrightarrow{{}}2K\] \[2K+2{{H}_{2}}O\xrightarrow{{}}2KOH+{{H}_{2}}\uparrow \] At anode.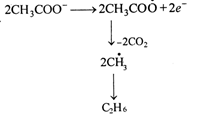
You need to login to perform this action.
You will be redirected in
3 sec
