| List - I | List -II |
| (A) \[BC{{l}_{3}}\] | (i) Linear |
| (B) \[Pdr_{4}^{2-}\] | (ii) Planar triangular |
| (C) \[S{{F}_{6}}\] | (iii) Tetrahedral |
| (D) \[\text{I}_{3}^{-}\] | (iv) Square planar |
A) A-(ii) B-(iii) C-(iv) D-(i)
B) A-(v) B-(iii) C-(ii) D-(i)
C) A-(ii) B-(v) C-(iv) D-(i)
D) A-(v) B-(iv) C-(iii) D-(ii)
Correct Answer: C
Solution :
\[BC{{l}_{3}}=3bp+0lp\] ie, hybridisation is \[s{{p}^{2}}\] and geometry is planar triangular. \[{{[PdB{{r}_{4}}]}^{2-}}\] The outer electronic configuration of Pd \[[Kr]\,4{{d}^{10}},5{{s}^{o}}.\] \[P{{d}^{2+}}=[Kr]4{{d}^{8}},5{{s}^{0}}\]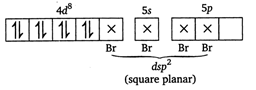 \[S{{F}_{6}}=6bp+olp\]ie, hybridization is \[s{{p}^{3}}{{d}^{2}}\]and the geometry is octahedral. \[\text{I}_{3}^{-}=2bp+3lp\]ie, hybridization is \[s{{p}^{3}}d\]but geometry is linear due to presence of 3 lone pair of electrons. Hence, \[A-(ii),\,B-(v),\,C-(iv),D-(i)\]
\[S{{F}_{6}}=6bp+olp\]ie, hybridization is \[s{{p}^{3}}{{d}^{2}}\]and the geometry is octahedral. \[\text{I}_{3}^{-}=2bp+3lp\]ie, hybridization is \[s{{p}^{3}}d\]but geometry is linear due to presence of 3 lone pair of electrons. Hence, \[A-(ii),\,B-(v),\,C-(iv),D-(i)\]
You need to login to perform this action.
You will be redirected in
3 sec
