A) \[NH3>{{H}_{2}}O>C{{O}_{2}}\]
B) \[C{{O}_{2}}>N{{H}_{3}}>{{H}_{2}}O\]
C) \[{{H}_{2}}O>C{{O}_{2}}>N{{H}_{3}}\]
D) \[{{H}_{2}}O>N{{H}_{3}}>C{{O}_{2}}\]
Correct Answer: D
Solution :
| | (due to regular geometry) |
| | (due to presence of two lone pair of electrons \[\mu \ne 0\]) |
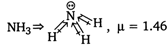 | (due to presence of one lone pair of electron\[\mu \ne 0\] ) |
You need to login to perform this action.
You will be redirected in
3 sec
