A) 12, 16
B) 12, 12
C) 8, 8
D) 12, 4
Correct Answer: A
Solution :
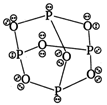 Structure of phosphorus trioxide \[({{P}_{4}}{{O}_{6}})\] Hence, \[{{P}_{4}}{{O}_{6}}\]molecule contains 12 P?O? bonds and 16 lone pair of electrons.
Structure of phosphorus trioxide \[({{P}_{4}}{{O}_{6}})\] Hence, \[{{P}_{4}}{{O}_{6}}\]molecule contains 12 P?O? bonds and 16 lone pair of electrons.
You need to login to perform this action.
You will be redirected in
3 sec
