A) I and II
B) II and III
C) II, III and IV
D) I, II, III and IV
Correct Answer: B
Solution :
\[\text{Xe}{{\text{O}}_{\text{3}}}\]is an explosive pyramidal molecule due to presence of one lone pair of electron.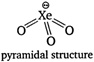 (II) In Fisher Ringes method, the oxygen and nitrogen of air are removed by passing it over a mixture of calcium carbide and calcium chloride. (Ill) He and Ne both have high ionization energy and lack of d-orbitals, so they are chemically inert. (IV) Except He all inert gases are adsorbed on activated charcoal and the extent of adsorption increases on moving down the group. Hence, only statement (II) and (III) are correct for noble gases.
(II) In Fisher Ringes method, the oxygen and nitrogen of air are removed by passing it over a mixture of calcium carbide and calcium chloride. (Ill) He and Ne both have high ionization energy and lack of d-orbitals, so they are chemically inert. (IV) Except He all inert gases are adsorbed on activated charcoal and the extent of adsorption increases on moving down the group. Hence, only statement (II) and (III) are correct for noble gases.
You need to login to perform this action.
You will be redirected in
3 sec
