A)
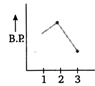
B)
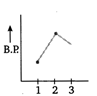
C)
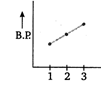
D)
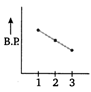
Correct Answer: C
Solution :
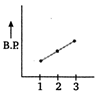 In both ethyl alcohol (2) and acetic acid (3) hydrogen bonding is present and the extent of H-bonding is more in acetic acid than ethyl alcohol due to formation of ring. Thus, acetic acid has higher b. p. than ethyl alcohol. Ethane (1) has lowest boiling point due to absence of B.P H-bonding. Hence, the graph showing correct order of boiling points is
In both ethyl alcohol (2) and acetic acid (3) hydrogen bonding is present and the extent of H-bonding is more in acetic acid than ethyl alcohol due to formation of ring. Thus, acetic acid has higher b. p. than ethyl alcohol. Ethane (1) has lowest boiling point due to absence of B.P H-bonding. Hence, the graph showing correct order of boiling points is
You need to login to perform this action.
You will be redirected in
3 sec
