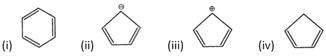
A) I, IV
B) I, II
C) III, IV
D) All of these
Correct Answer: C
Solution :
According to Huckel s rule for aromaticity, a compound must follow following rules : (i) It must be cyclic and planar. (ii) It must have a conjugated system in it (iii) It must have \[(4n+2)\pi \] n electrons. (i) In, no. of \[\pi \]electrons \[=4+2=6\] \[(4n+2)=(4\times 1+2)=6,\]thus Huckels rule is followed.
no. of \[\pi \]electrons \[=4+2=6\] \[(4n+2)=(4\times 1+2)=6,\]thus Huckels rule is followed. 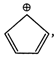 no. of \[\pi \]electrons = 4 \[(4n+2)\ne 4,\]thus Huckles rule is not followed
no. of \[\pi \]electrons = 4 \[(4n+2)\ne 4,\]thus Huckles rule is not followed You need to login to perform this action.
You will be redirected in
3 sec
