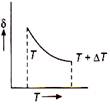
A)
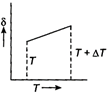
B)
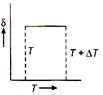
C)
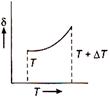
D)
Correct Answer: C
Solution :
From ideal gas equation \[pV=RT\] or \[p\Delta V=R\Delta T\] Dividing eq. (ii) by eq. (i), we get \[\frac{\Delta V}{V}=\frac{\Delta T}{T}\] \[\frac{\Delta V}{V\Delta T}=\frac{1}{T}=\delta \] \[\therefore \] \[\delta =\frac{1}{T}.\] So, the graph between \[\delta \] and T will be rectangular graph.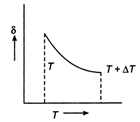
You need to login to perform this action.
You will be redirected in
3 sec
