A) 3
B) 4
C) 5
D) 6
Correct Answer: C
Solution :
Since, the coordination number of Co is 6, there must be six ligands inside the coordination sphere. As one mole of complex give three moles of ions, two \[\text{C}{{\text{l}}^{-}}\]ions must present outside the sphere. Hence, the formula of the complex is \[[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}.[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}\]\[\xrightarrow{\text{Ionisation}}\underbrace{{{[Co{{(N{{H}_{3}})}_{5}}Cl]}^{2+}}+2\text{C}{{\text{l}}^{-}}}_{\text{3 }\,\text{ions}}\] In this complex one Cl atom satisfies both primary and secondary valencies as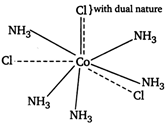 Hence, the value of Y is 5.
Hence, the value of Y is 5.
You need to login to perform this action.
You will be redirected in
3 sec
